Diamond opposite on mohs scale – In the realm of minerals, the Mohs scale of hardness reigns supreme, and diamond stands as the undisputed champion at the apex. But what lies at the opposite end of this spectrum, a mineral so soft it’s the polar opposite of diamond’s unyielding nature? Embark on a journey to discover the fascinating world of minerals with a Mohs hardness of 1, the antithesis of diamond.
These exceptionally soft minerals, a stark contrast to diamond’s unwavering rigidity, possess unique characteristics and play significant roles in various industries. Join us as we delve into the captivating realm of diamond’s opposite on the Mohs scale, exploring their properties, applications, and the intriguing reasons behind their contrasting hardness.
Introduction

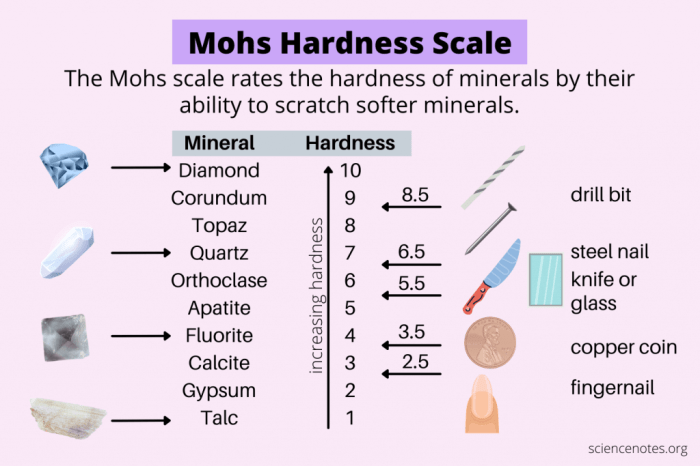
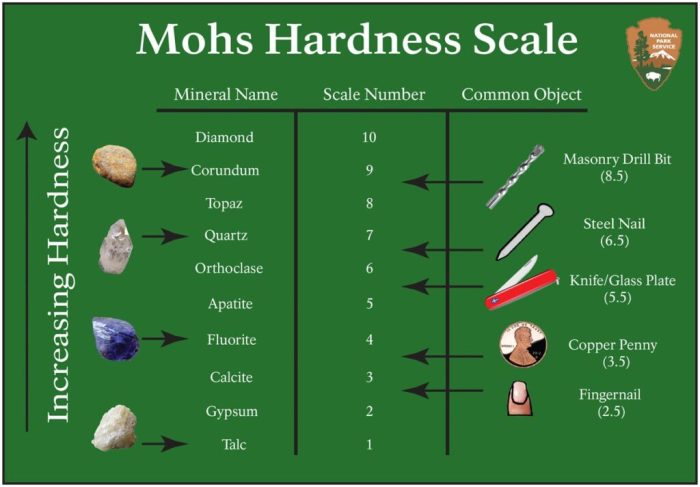
The Mohs scale of mineral hardness is a qualitative measure of the resistance of a mineral to scratching. It was developed by the German mineralogist Friedrich Mohs in 1822 and is based on the ability of one mineral to scratch another.
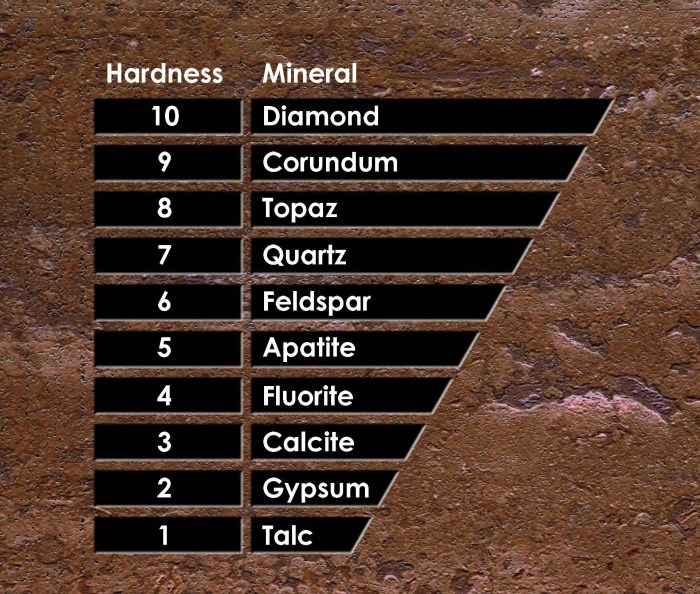
The scale consists of 10 minerals, with each mineral being harder than the one below it. The minerals are:
- 1. Talc
- 2. Gypsum
- 3. Calcite
- 4. Fluorite
- 5. Apatite
- 6. Orthoclase
- 7. Quartz
- 8. Topaz
- 9. Corundum
- 10. Diamond
Diamond is the hardest mineral on the Mohs scale, with a hardness of 10. This means that it cannot be scratched by any other mineral.
Diamond is a crystalline form of carbon. It is the hardest known natural material and is used in a variety of industrial and commercial applications, including cutting tools, abrasives, and jewelry.
Minerals Opposite Diamond on the Mohs Scale
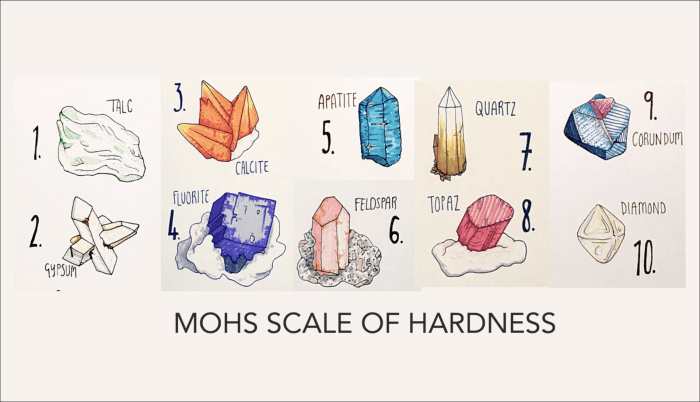
At the opposite end of the Mohs scale from diamond, with a hardness of 10, lie minerals with a hardness of 1. These minerals are exceptionally soft and can be easily scratched by a fingernail.
Talc
- Talc is a hydrated magnesium silicate mineral that is often used as a filler in cosmetics, paints, and ceramics.
- It has a greasy feel and a pearly luster.
- Talc is also used as a lubricant and an anti-caking agent in food products.
Graphite
- Graphite is a form of carbon that is composed of stacked layers of graphene.
- It is a soft, black mineral that is used as a lubricant, a pencil lead, and an electrode in batteries.
- Graphite is also a good conductor of electricity.
Mica
- Mica is a group of minerals that are composed of layered silicates.
- It is a soft, flaky mineral that is used in a variety of applications, including as a filler in paints, plastics, and roofing materials.
- Mica is also used as a lubricant and an electrical insulator.
Comparison of Diamond and Minerals Opposite on the Mohs Scale
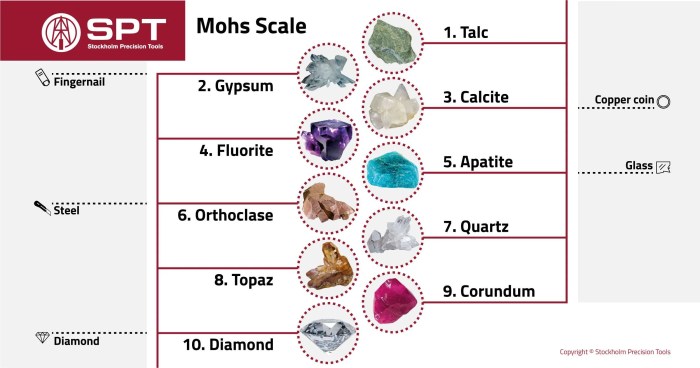
Diamond, with a Mohs hardness of 10, stands at the pinnacle of hardness, while minerals with a Mohs hardness of 1, such as talc, occupy the opposite end of the spectrum. This stark contrast in hardness is accompanied by a range of distinct physical properties.
Hardness
Diamond’s exceptional hardness arises from its unique atomic structure. Its carbon atoms form a rigid, three-dimensional lattice, resulting in extremely strong atomic bonds. In contrast, minerals with a Mohs hardness of 1, such as talc, have weak interatomic bonds, making them easily scratched or powdered.
Luster
Diamond exhibits an adamantine luster, characterized by its intense brilliance and high refractive index. Minerals with a Mohs hardness of 1, such as talc, typically have a dull, earthy luster due to their low refractive index and the presence of impurities.
Diamond, the hardest known material, is at the opposite end of the Mohs scale from graphite, the softest. This vast difference in hardness is due to the arrangement of their atoms. Diamond’s atoms are held together by strong covalent bonds, while graphite’s atoms are held together by weaker ionic bonds.
For more information on ionic bonds, check out the ionic bonds gizmos answer key . The strength of diamond’s covalent bonds makes it resistant to scratching and wear, making it ideal for use in cutting tools and jewelry.
Cleavage
Diamond possesses perfect octahedral cleavage, meaning it tends to break along eight specific planes. Minerals with a Mohs hardness of 1, such as talc, have poor to absent cleavage, making them more likely to crumble or powder rather than break along specific planes.
Applications of Diamond and Minerals Opposite on the Mohs Scale
Diamonds and minerals with a Mohs hardness of 1 possess unique properties that lend them to a wide range of practical applications across various industries and scientific fields.
Applications of Diamond, Diamond opposite on mohs scale
Diamond’s exceptional hardness makes it an indispensable material in industries that require extreme durability and precision.
- Cutting Tools:Diamond-tipped cutting tools are used in industries such as construction, manufacturing, and dentistry, where they provide superior cutting performance and extended tool life.
- Abrasives:Diamond powder is employed as an abrasive in grinding and polishing applications, offering unmatched cutting efficiency and surface finishing.
- Jewelry:Diamonds are highly valued in jewelry for their brilliance, durability, and cultural significance.
- Industrial Coatings:Diamond-like carbon (DLC) coatings are applied to surfaces to enhance their hardness, wear resistance, and corrosion protection.
Applications of Minerals with Mohs Hardness of 1
Minerals with a Mohs hardness of 1, such as talc and graphite, find uses in various industries and scientific fields due to their unique properties.
- Lubricants:Talc powder is widely used as a dry lubricant in industries such as food processing, pharmaceuticals, and plastics manufacturing.
- Cosmetics:Talc is a common ingredient in cosmetics, providing a smooth and velvety texture.
- Electronics:Graphite is used as an electrical conductor in batteries, electrodes, and electronic devices.
- Pencils:Graphite is the primary component of pencil lead, providing a smooth and dark writing surface.
Conclusion
In summary, diamond and minerals opposite on the Mohs scale possess unique properties and applications. Diamond, with its exceptional hardness and thermal conductivity, finds use in various industries, including cutting tools, abrasives, and jewelry. On the other hand, minerals like talc and graphite, being soft and slippery, are employed as lubricants, fillers, and pigments.
Understanding the contrasting properties of these materials allows for tailored applications and advancements in materials science.
Future research directions may explore the development of synthetic diamond substitutes with comparable or even superior properties, expanding their industrial applications. Additionally, the study of minerals opposite on the Mohs scale could lead to novel materials with unique properties for use in diverse fields such as energy storage and biomedicine.
FAQ Overview: Diamond Opposite On Mohs Scale
What is the Mohs scale of hardness?
The Mohs scale is a qualitative measure of the scratch resistance of minerals, ranging from 1 (softest) to 10 (hardest).
What is the significance of minerals with a Mohs hardness of 1?
Minerals with a Mohs hardness of 1 are exceptionally soft and can be easily scratched by a fingernail. They often have layered structures and are used in various applications, including art, cosmetics, and medicine.
How do minerals with a Mohs hardness of 1 compare to diamond?
Diamond, with a Mohs hardness of 10, is the hardest known natural material. Minerals with a Mohs hardness of 1 are at the opposite end of the spectrum, making them extremely soft in comparison.
What are some examples of minerals with a Mohs hardness of 1?
Talc, gypsum, and graphite are examples of minerals with a Mohs hardness of 1.