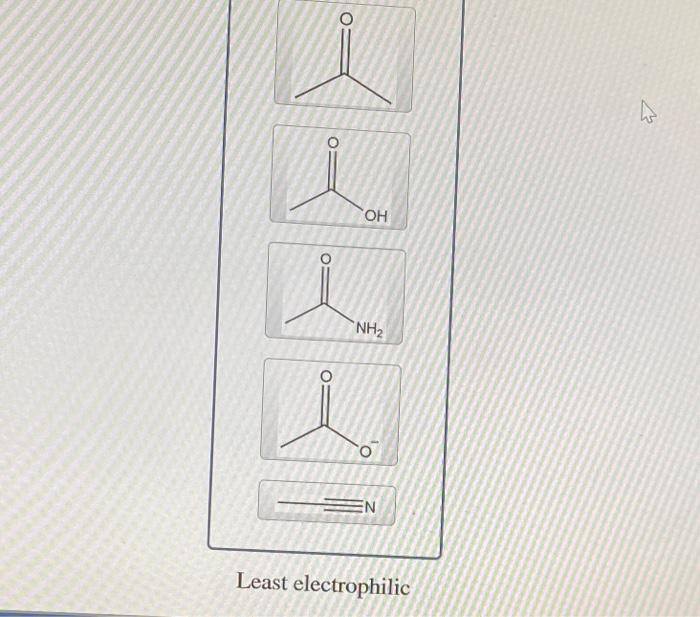
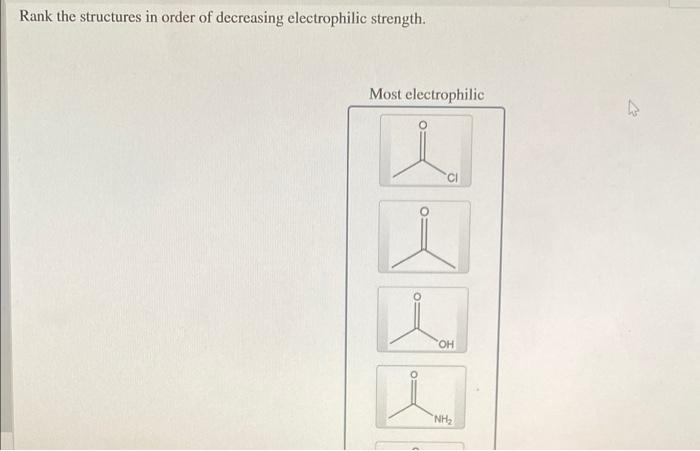
Rank the structures in order of decreasing electrophilic strength. – In the realm of organic chemistry, electrophilic strength plays a pivotal role in determining the reactivity of carbocations. This article delves into the concept of electrophilic strength, exploring its relationship with carbocation stability and the factors that influence it, including resonance, inductive effects, and steric hindrance.
Rank the Structures in Order of Decreasing Electrophilic Strength: Rank The Structures In Order Of Decreasing Electrophilic Strength.
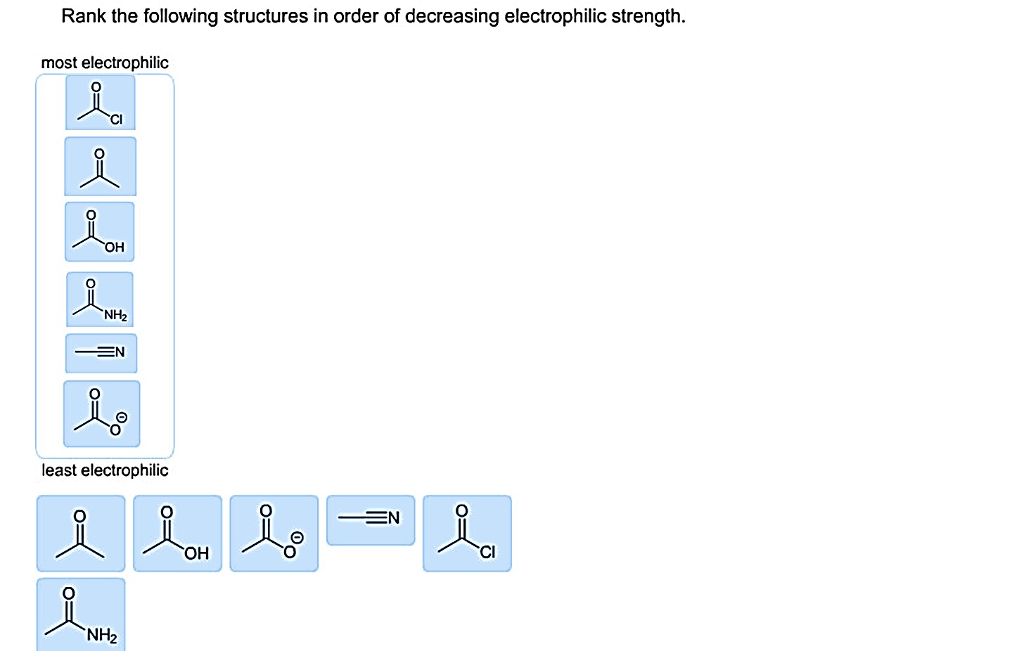
Electrophilic strength refers to the ability of a chemical species to attract electrons. In the context of carbocations, electrophilic strength is directly related to the stability of the carbocation. A more stable carbocation is less electrophilic because it is less likely to react with nucleophiles.
The stability of a carbocation is determined by the number of alkyl groups attached to the positively charged carbon atom. The more alkyl groups that are attached, the more stable the carbocation. This is because the alkyl groups donate electrons to the positively charged carbon atom, which helps to stabilize the charge.
Based on the above discussion, we can rank the following carbocations in order of decreasing electrophilic strength:
- CH3+
- CH 3CH 2+
- CH 3CH 2CH 2+
CH 3+is the most electrophilic because it has no alkyl groups to donate electrons to the positively charged carbon atom. CH 3CH 2+is less electrophilic because it has one alkyl group to donate electrons to the positively charged carbon atom.
CH 3CH 2CH 2+is the least electrophilic because it has two alkyl groups to donate electrons to the positively charged carbon atom.
Questions and Answers
What is electrophilic strength?
Electrophilic strength refers to the ability of a species to accept electrons.
How does resonance affect electrophilic strength?
Resonance can stabilize carbocations, reducing their electrophilic strength.
How do inductive effects influence electrophilic strength?
Inductive electron-withdrawing groups increase electrophilic strength, while electron-donating groups decrease it.
What is the role of steric hindrance in electrophilic strength?
Steric hindrance can hinder the approach of nucleophiles, reducing electrophilic strength.